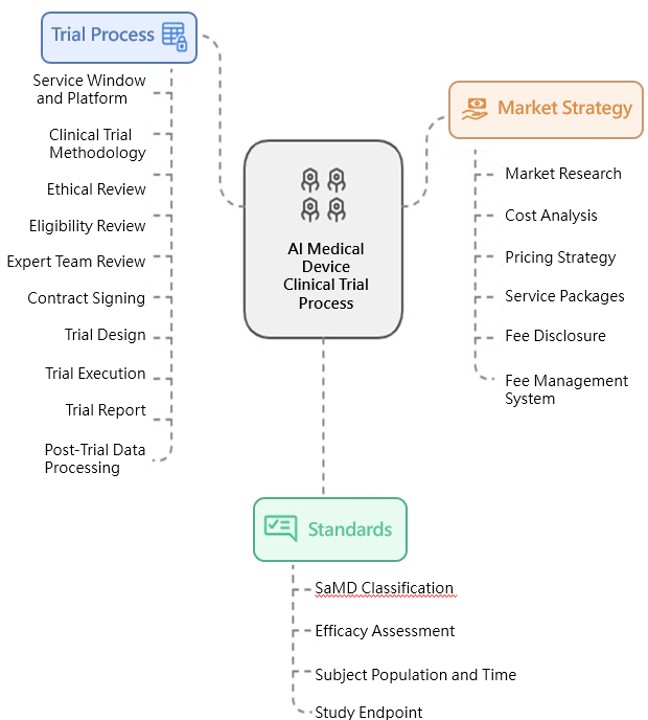
The AI Impact Research Center of Taipei Veterans General Hospital assists AI medical devices (AI-SaMD) that have obtained Taiwan FDA approval in conducting clinical trials, aiming to obtain comprehensive health impact and medical economic assessments, with the ultimate goal of helping products apply for national health insurance coverage. The following details the trial process, standards, and market strategies.
I. Trial Process
0506.jpg)
0506.jpg)
II. Standards
0506.jpg)
III. Market Strategy
The market strategy services provided by the center are as follows:
0506.jpg)
Last updated: 2025/05/06 15:40:00