Core Mission
Assist AI medical devices (AI-SaMD) that have obtained certification to conduct clinical trials, obtain comprehensive health impact and medical economic assessments, and apply for health insurance coverage.
Application Qualifications
- AI products must pass TFDA registration and inspection.
- AI products must comply with the scope of medical device software applicable to the "Technical Guidelines for Registration and Inspection of Medical Device Software with Artificial Intelligence/Machine Learning Technology" announced by TFDA.
Application Steps
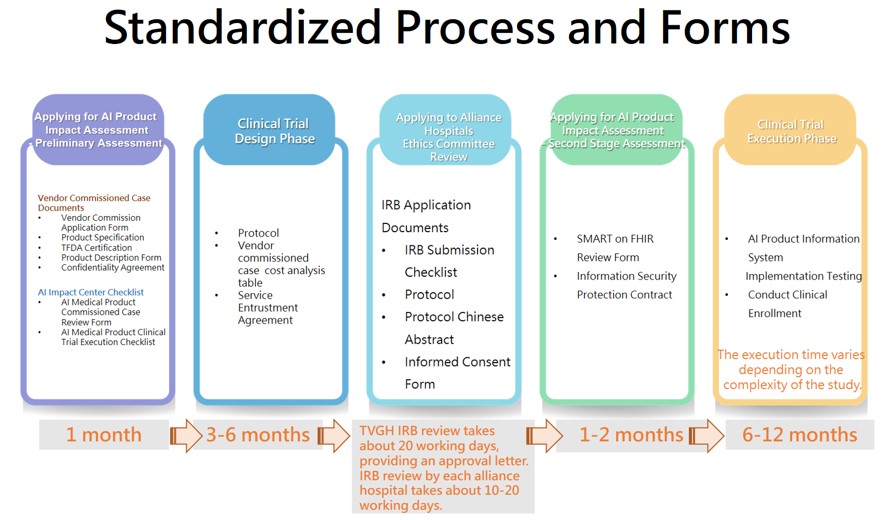
- Fill out the Application Form
- After our center responds, please refer to the application process diagram, prepare the relevant documents, download the designated forms from the section below, fill them out, and send them to AICENTER.TVGH@gmail.com (Subject: XX Company-Application Request)
Application Document Download Area
Non-Disclosure Agreement
TVGH AI Impact Research Center_Non-Disclosure Agreement_20250315 v1.1
DownloadProduct Description Form
TVGH AI Impact Research Center_Product Description Form_20250310 v1.0
DownloadSMART on FHIR Standard Application Checklist
TVGH AI Impact Research Center_SMART on FHIR Standard Application Checklist_20250310 v1.0
DownloadCommission Contract
TVGH AI Medical Device Software Impact Assessment Commission Contract_20250316_v1.1
DownloadInformation Security Protection Contract
TVGH AI Impact Research Center_Information Security Protection Contract_20250310 v1.0
DownloadCommission Progress Inquiry
Please enter the case number to check the commission progress:
Last updated: March 16, 2025 16:40:32